Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multi-center placebo-controlled randomized clinical trial
BACKGROUND No definitive treatment exists for Coronavirus Disease 2019 (COVID-19). Honey and Nigella sativa (HNS) have established antiviral, antibacterial, anti-inflammatory and immunomodulatory properties. Hence, we investigated efficacy of HNS against COVID-19.
METHODS We conducted a multicenter, placebo-controlled, randomized clinical trial at 4 centers in Pakistan. RT-PCR confirmed COVID-19 adults showing moderate or severe disease were enrolled in the study. Patients presenting with multi-organ failure, ventilator support, and chronic diseases (except diabetes mellitus and hypertension) were excluded. Patients were randomly assigned in 1:1 ratio to receive either honey (1 gm/Kg/day) and Nigella sativa seeds (80 mg/Kg/day) or placebo up-to 13 days along with standard care. The outcomes included symptom alleviation, viral clearance, and a 30-day mortality in intention-to-treat population. This trial was registered with ClinicalTrials.gov, NCT04347382.
PROCEDURE The HNS group received honey (1 gm) plus encapsulated Nigella sativa seeds (80 mg) per kg body weight orally in 2-3 divided doses daily for up-to 13 days while the control group received placebo (empty capsules). Both the products were certified for purity by botany department of Government College University, Lahore, Pakistan. Additionally, each patient in the trial received standard care therapy (SCT) as recommended by the treating physician and the clinical management guidelines for COVID-19 established by the Ministry of National Health Services of Pakistan... this version posted November 30, 2020. The copyright holder for this comprised of antipyretics, antibiotics, anticoagulants, steroids, supplemental oxygen and mechanical ventilation. The study participants were assessed for clinical symptoms daily by an onsite investigator for 13 days.
NICE and Public Heath England guidelines also recommend honey as the first line of treatment for an acute cough caused by upper respiratory tract infections, known as one of the defining symptoms of COVID-19. These findings strengthen the use of HNS as a potential candidate for combating SARS-CoV-2 worldwide.
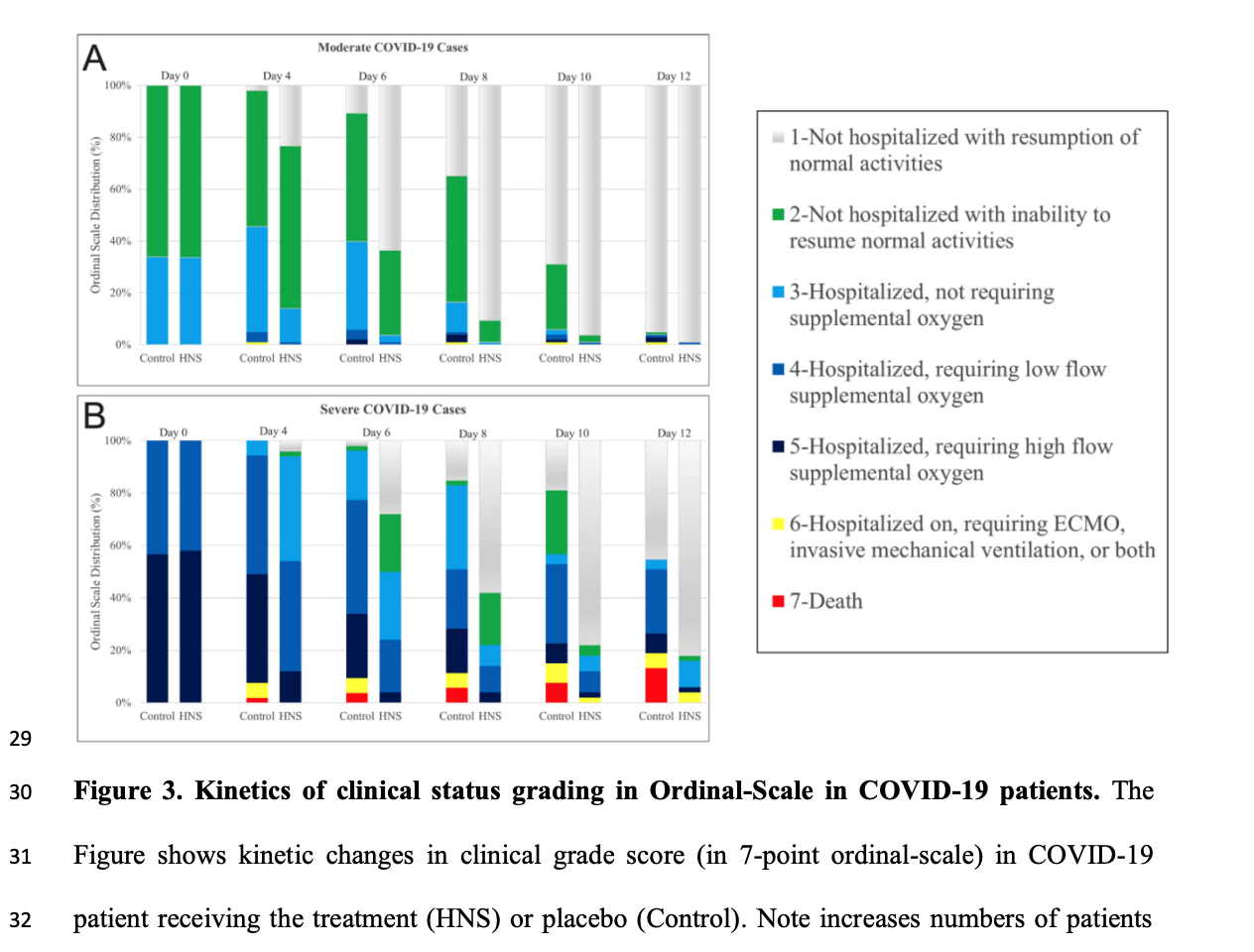
The recovery time reported for remdesivir was 10 days versus 15 days for the control (P<0.001),3 whereas lopinavir-ritonavir resulted in no decrease in the recovery time (16 days versus 16 days; P=0.09).2 In our study, in ~50% of cases, SARS-CoV-2 RT-PCR became negative 4 days sooner in HNS groups than control groups. In previous studies, mortality among severe cases in comparison to control group was 27.0% (versus 25.0%) for hydroxychloroquine,1 19.2% (versus 25.0%) for lopinavir-ritonavir,2 15.7% (versus 24.0%) for convalescent plasma,2811.4% (versus 15.2%) for remdesivir,3 22.9% (versus 25.7%) for dexamethasone,4 and only 4% (versus 18.87%) for HNS. Thus, HNS provided clinical superiority in reducing mortality in COVID-19 patients. Of note, combined mortality data provided by Solidarity and ACTT-1 for remdesivir and by Solidarity and Recovery trial for lopinavir-ritonavir failed to provide statistical improvement in mortality.29 In contrast to these drugs, HNS represents a safer and more affordable option that can be used as an in-house remedy.
https://www.medrxiv.org/content/10.1101/2020.10.30.20217364v4.full.pdf+html
Nigella sativa
Honey and Nigella sativa against COVID-19 in Pakistan (HNS-COVID-PK): A multi-center placebo-controlled randomized clinical trial.
To the best of our knowledge, this study is the first randomized, placebo- controlled clinical trial assessing the efficacy of oral honey and Nigella sativa seeds among adults with moderate or severe COVID-19. In the intention-to-treat analysis, we provided comprehensive methodical descriptions of clinical parameters, and clinical outcomes. In COVID-19 patients, honey and Nigella sativa with standard care therapy resulted in earlier viral clearance, symptomatic relief, clinical improvement and mortality reduction. Moreover, similarly to previous research no adverse effects were reported regarding HNS.
Considering the economic crisis related to the COVID-19 pandemic, the use of honey and Nigella sativa will particularly be beneficial for impoverished populations in resource limited settings. The inexpensive over the counter treatment regimen would be a valuable source to lower the burden on healthcare system while significantly dampening impact of the disease.
Fanciful is the idea that the world's oligarchs, as they try to impose their vaccines on us, would be at all concerned about the problems of "populations in resource limited settings"--unless the idea is to squeeze pennies from billions.
Research[edit]
One meta-analysis of clinical trials found weak evidence that N. sativa has a short-term benefit on lowering systolic and diastolic blood pressure, with limited evidence that various extracts of black seed can reduce triglycerides and LDL and total cholesterol, while raising HDL cholesterol.[16]
Unwelcome evidence is always weak.
Frontiers in Cellular - and Infection Microbiology
Multidisciplinary open-access journal led by Field Chief Editor Yousef Abu Kwaik frontiersin.org
Abstract
The world is witnessing a difficult time. The race of developing a new coronavirus (COVID-19) vaccine is becoming more urgent. Many preliminary studies on the pathophysiology of COVID-19 patients have provided some clues to treat this pandemic. However, no suitable treatment has found yet. Various symptoms of patients infected with COVID-19 indicated the importance of immune regulation in the human body. Severe cases admitted to the intensive care unit showed high level of pro-inflammatory cytokines which enhanced the disease severity. Acute Respiratory Distress Syndrome (ARDS) in COVID-19 patients is another critical factor of disease severity and mortality. So, Immune modulation is the only way of regulating immune system. Nigella sativa has been used for medicinal purposes for centuries. The components of this plant are known for its intense immune-regulatory, anti-inflammatory, and antioxidant benefits in obstructive respiratory disorders. A molecular docking study also gave evidences that N. sativa decelerates COVID-19 and might give the same or better results than the FDA approved drugs. The aim of this review was to investigate the possible immune-regulatory effects of N. sativa on COVID-19 pandemic. Our review found N. sativa's Thymoquinone, Nigellidine, and α-hederin can be a potential influencer in reinforcing the immune response on molecular grounds.
Despite considerable use of N. sativa in traditional medicine practices in Africa and Asia, there is insufficient high-quality clinical evidence to indicate that consuming the seeds or oil provides any benefit to human health.[4]
Thymoquinone
| Thymoquinone is a phytochemical compound found in the plant Nigella sativa. It is also found in select cultivated Monarda fistulosa plants which can be steam distilled to produce an essential oil. |
It has been classified as a pan-assay interference compound, which binds indiscriminately to many proteins.[1] It is under preliminary research to identify its possible biological properties.[2]
- Baell JB (March 2016). "Feeling Nature's PAINS: Natural Products, Natural Product Drugs, and Pan Assay Interference Compounds (PAINS)". Journal of Natural Products. 79 (3): 616–28. doi:10.1021/acs.jnatprod.5b00947. PMID 26900761.
Farkhondeh T, Samarghandian S, Borji A (September 2017). "An overview on cardioprotective and anti-diabetic effects of thymoquinone". Asian Pacific Journal of Tropical Medicine. 10 (9): 849–854. doi:10.1016/j.apjtm.2017.08.020. PMID 29080612.
The idea of using herbal medicine is totally alien and it is even more abhorrent to Pharma than out-of-patent medications.
If even product of Pharma, like HCQ and ivermectin are unacceptable to our Dear Leaders, how much more hateful to them can the thought be, of allowing people to take herbal medications to cure or prevent an illness that has created billions of profits to the covid profiteers and millions to their enablers.




